

Programs
Innovative therapies
for more patients.
We are committed to developing first- and best-in-class therapies for patients with high unmet need across a wide range of diseases.

Therapeutic Areas of Focus
Broad Pipeline of First – and Best-in-Class Medicines
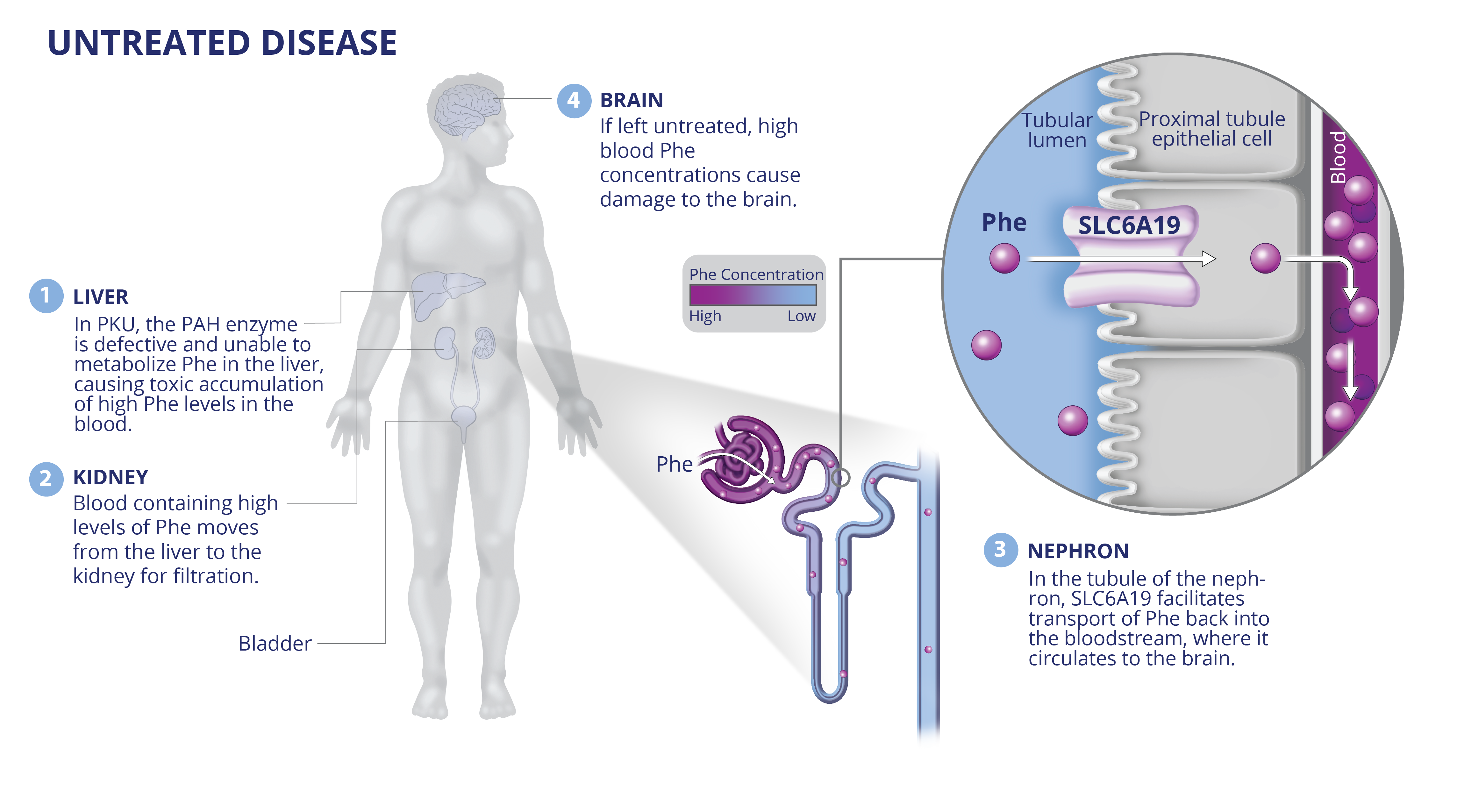
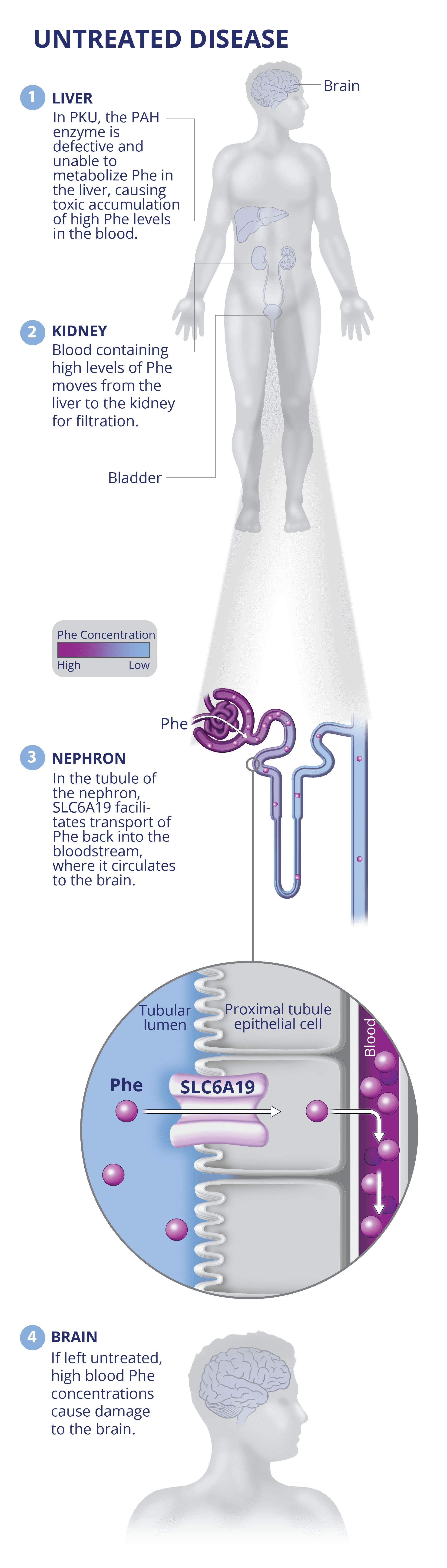
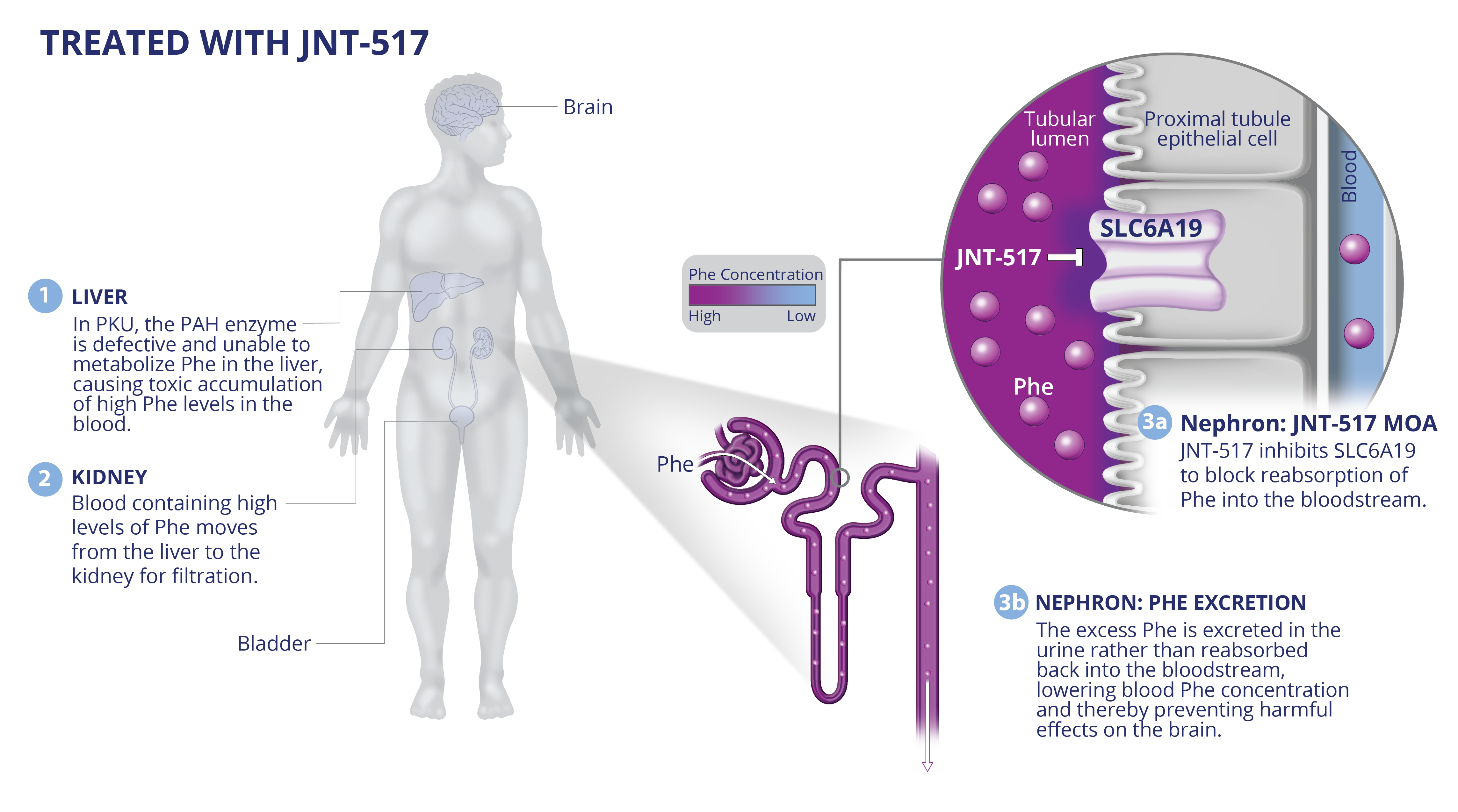
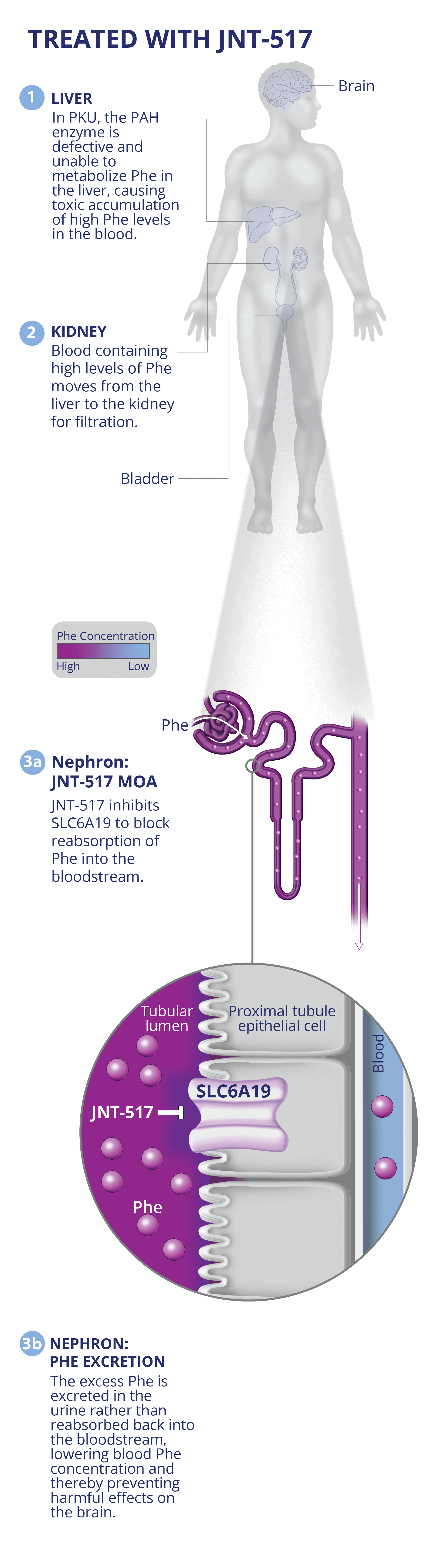
PKU

JNT-517: First-in-class discovery program against validated SLC6A19 target in PKU.
JNT-517 was derived from an allosteric inhibitor series identified by RAPID. A proprietary cryo-EM structure of SLC6A19 in complex with a Jnana proprietary compound has revealed that Jnana’s allosteric inhibitors bind in an unprecedented cryptic allosteric site on the SLC transporter.
Immune-Mediated Diseases


Potential first-in-class discovery program against validated IRF3 target.
We are advancing the first reported ligands targeting IRF3. Interferon regulatory factor 3 (IRF3) is a well-validated downstream target of both the STING and MAVS pathways. IRF3 has been identified as a promising, high-value target with implications across several autoimmune and inflammatory disorders – but like many transcription factors, efforts to identify small molecule ligands for IRF3 have not yet been successful, possibly due to an absence of structured binding pockets.
With our RAPID platform, we have identified RAPs with significant selectivity for IRF3, conducted a high throughput screen and identified drug-like small molecules that displace a RAP and bind IRF3. We have solved the x-ray crystal structure of both a RAP fragment and a HTS hit in complex with the protein; these are the first known ligands targeting IRF3.


Collaborations

