

Clinical Trial for
INDIVIDUALS WITH PHENYLKETONURIA (PKU)
NOW ENROLLING.
Jnana Therapeutics is committed to developing new therapies in areas of high unmet medical need.


Our team of scientists and clinicians have identified and are studying a new investigational medication called JNT-517 for the potential treatment of PKU.
Due to the challenge of adhering to the PKU diet and limited treatment options, some individuals with PKU have phenylalanine (Phe) levels that exceed the recommended range leading to symptoms such as difficulty with memory/thinking, depression, anxiety and poor quality of life. New therapies are needed that are safe, convenient, and allow for Phe reduction and increased natural protein intake.

PKU Phase 1b Trial
Jnana has completed a clinical study of the JNT-517 medication in individuals without PKU and is now conducting a clinical study in individuals with PKU to determine:
The safety of the medication and how well it’s tolerated
How the medication affects Phe levels in the urine and blood
Jnana’s Medication (JNT-517)
Will be taken by mouth
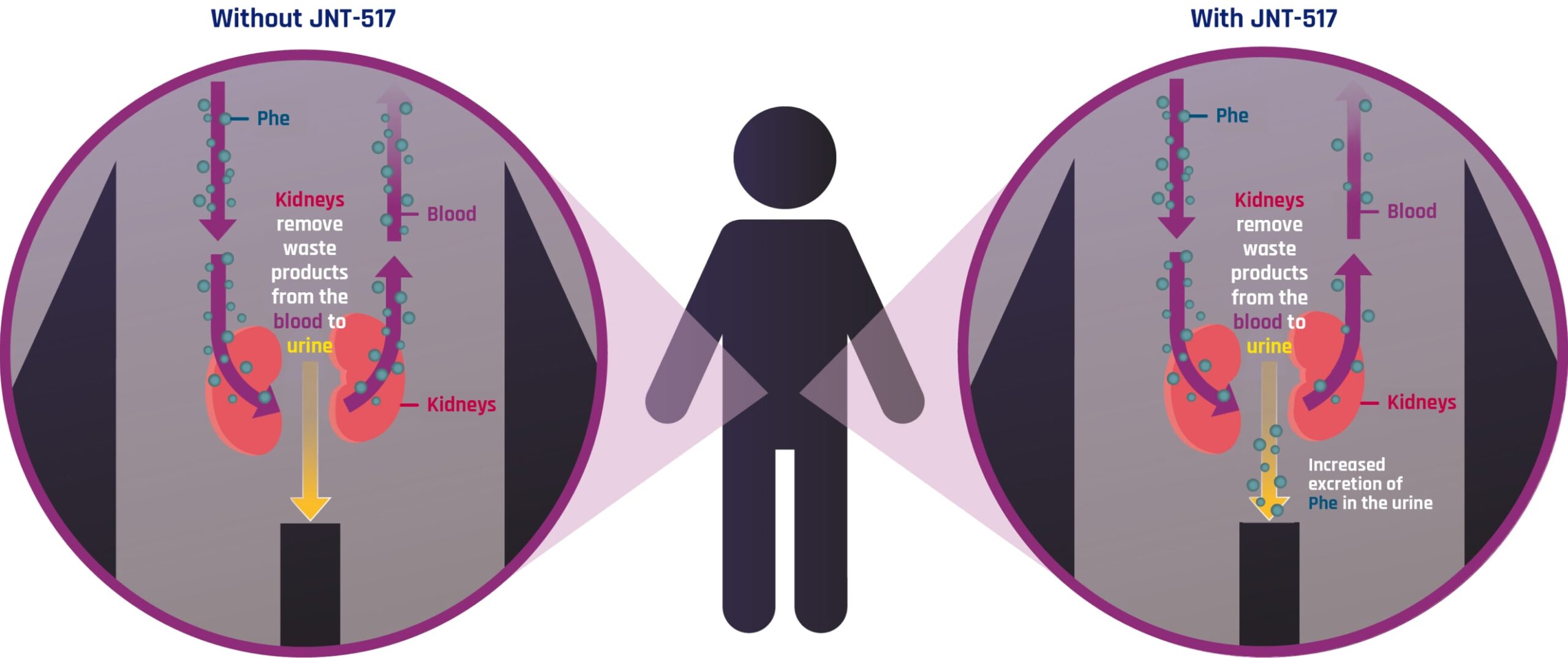
Designed to help the body get rid of excess Phe by:
Blocking Phe reabsorption from the kidney back into the blood
Increasing excretion of Phe in the urine
Eligibility
To qualify, patients must:
Have PKU diagnosis
Be adults (ages 18-65 years)
Have at least 2 blood Phe values > 600µM or 10mg/dL in the past year
Currently not taking any PKU medications (i.e., Palynziq, Kuvan) or large neutral amino acid supplements or willing to stop taking these before participating in the study (consult with your physician before stopping any medications or supplements)
Not participating in other clinical studies of investigational treatments
No medical conditions that would limit participation
Who should I contact if I’m interested in participating in the trial or for more information?
If you are interested in participating in the trial, please discuss whether you qualify with your clinician.
For more information about the study, please see clinicaltrials.gov (NCT05781399) or contact [email protected]
